CAPA Management Software as Part of an Integrated QMS
Streamline investigations and close CAPAs faster with a SharePoint-based QMS built for regulated industries.

What is CAPA Software?
Why Choose BizPortals QMS’s CAPA Software?







BizPortals QMS allows users to quickly report, log, and track issues, including process deviation, incidents, non-conformances, and more in a centralized system and ensures that they are managed, resolved, and documented efficiently.
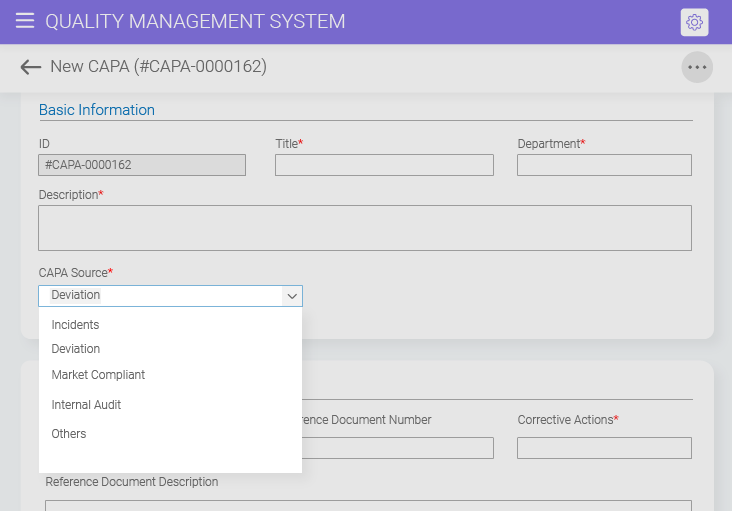
Cross-module integration facilitates holistic issue resolution by integrating workflows and data across different modules, such as audits, incidents, risks, and document control, improving the overall CAPA process flow.
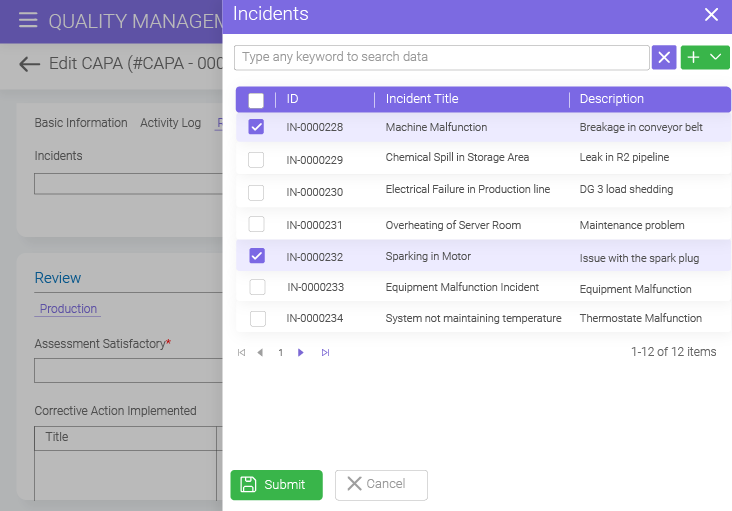
From reports to audit trials and more, BizPortals CAPA system offers a centralized place to access all the CAPA documentation. Dedicated document control features like version control, track changes, and role-based permissions help maintain transparency and compliance.
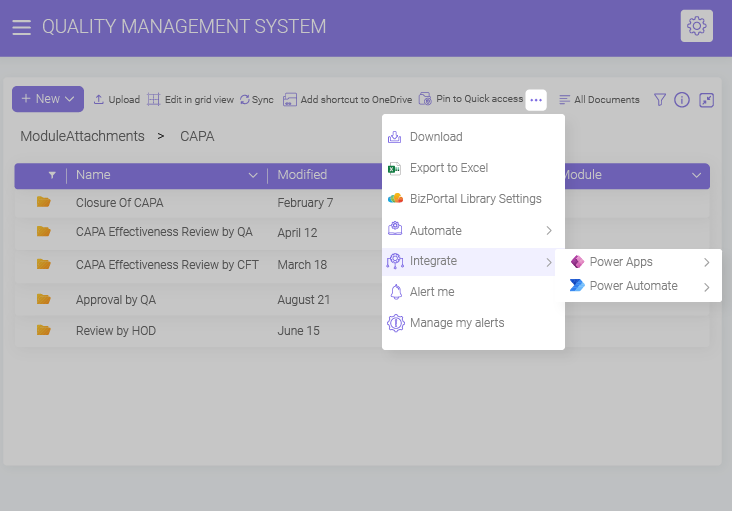
Leverage automated workflows for document routing, approval, and email reminders to effortlessly streamline various CAPA stages to ensure consistency across the organization and speed up the overall quality process.
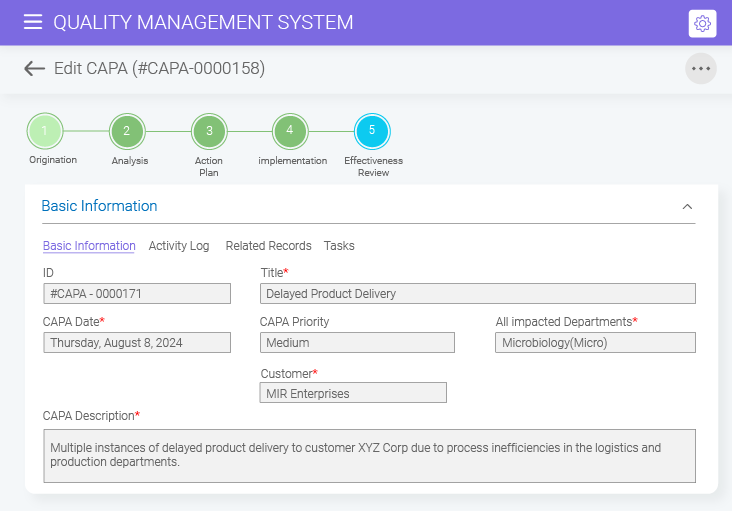
BizPortals QMS offers detailed activity logs to record issues and processes, interactive dashboards to identify trends and monitor progress, and data export features to ensure data visibility and facilitate sharing.
Easily configure CAPA workflow, define stakeholder’s roles, or create custom CAPA forms to capture essential details such as issue description, assessment details, root cause analysis, and corrective actions to streamline your quality improvement processes.
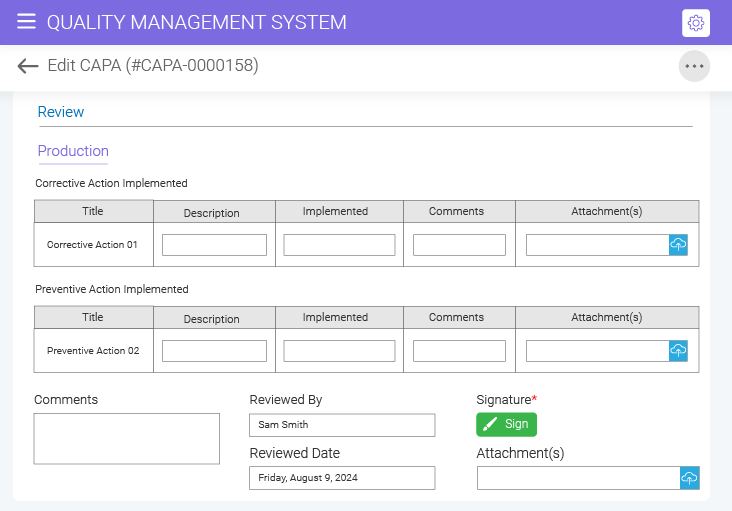
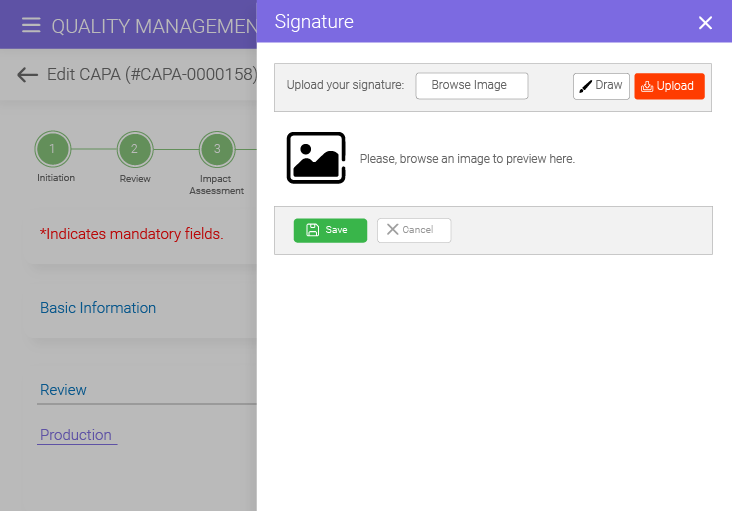
Effortlessly approve your CAPA documents with the e-signature feature offered by the BizPortals CAPA management solution. Simply draw and upload signatures or leverage Active Directory-based authentication for a detailed audit trail, enhanced transparency, and improved cost efficiency.
-
Issue Reporting and Tracking
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More -
Cross-Module Integration
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More -
Centralized CAPA Records
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More -
Automated CAPA Processes
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More -
Data Visibility
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More -
Configurable Processes and Forms
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More -
Faster Approvals with E-Signature
-->
A comprehensive solution to streamline business processes and manage all your documents, projects, tasks, and employees together in a secure workplace environment.
Read More
See the Full QMS in Action
Key Benefits of Using CAPA Software in Quality Processes

Enhanced Quality
CAPA plays a crucial role in quality management by systematically addressing the underlying issues, reducing the overall defect rates, and achieving higher quality outcomes.

Regulatory Compliance
Regulatory bodies like the FDA, ISO, and industry-specific agencies mandate CAPA. Hence, implementing a well-planned CAPA system facilitates smoother audits and inspections.

Risk Management
CAPA processes not only identify risks associated with the quality process but also facilitate proactive monitoring of potential risks causing disruption in quality operations.

Continuous Improvement
CAPA management software enables businesses to gather, analyze, and monitor trends associated with the issues, helping them identify key areas of improvement and process optimization.

Documentation and Traceability
Right from detecting issues to executing corrective and preventive actions, each stage is documented and records stakeholders’ actions, providing a clear audit trail for inspections.
Documentation and Traceability
How CAPA Management Software in BizPortals QMS Works: A Complete Process Overview
Issue Logging
Reporting an issue is the first step that initiates the CAPA procedure. BizPortals CAPA management software offers a comprehensive form for issue logging. With customizable fields to enter basic information including the CAPA title, description, sources, and more, it allows users to add attachments for a clear record of the issue.
Risk Assessment
Once the issue has been logged, assessing its impact on the overall product or services is essential. Automated risk assessment forms, dedicated approval workflows with multiple approvers, and a detailed audit trail of activities can help users effectively collaborate in the risk assessment process.
Root Cause Investigation
Identifying the root cause is the next crucial step in preventing the recurrence of such issues. BizPortals CAPA management software, with robust document control and collaborative features, ensures that users have access to all the CAPA records, audit logs, and documentation ready for the root cause analysis.
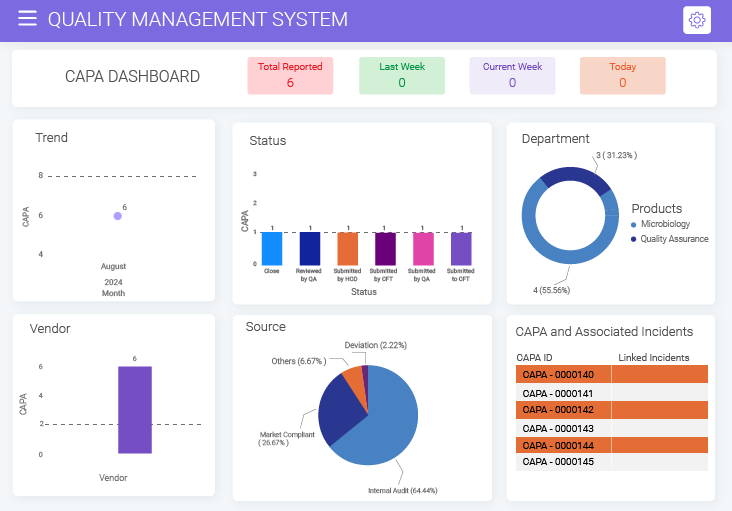
Trend Analysis
Analyzing previous CAPA trends by studying detailed statistics and metrics helps outline more concrete corrective actions. BizPortals QMS's dedicated CAPA dashboard displays key metrics based on recurring issues, potential risks, and key categories like monthly trends, departments, stages, priority, etc., and helps businesses refine their processes.
Corrective Action Planning
A robust corrective action plan is necessary to address the issues based on the root cause assessment and an in-depth trend analysis. Here, automated review and approval workflow, centralized quality resources, and cross departmental collaboration offered by SharePoint-based CAPA management software can be game changers
Implementing CAPA
Seamless execution and timely monitoring of the action plan decide the success of the overall corrective and preventive actions. This requires uninterrupted communication across departments, robust task management, progress tracking, and cross-module integration to ensure the plan is executed promptly and efficiently.
How CAPA Management Software in BizPortals QMS Works: A Complete Process Overview
Issue Logging
Reporting an issue is the first step that initiates the CAPA procedure. BizPortals CAPA management software offers a comprehensive form for issue logging. With customizable fields to enter basic information including the CAPA title, description, sources, and more, it allows users to add attachments for a clear record of the issue.
Risk Assessment
Once the issue has been logged, assessing its impact on the overall product or services is essential. Automated risk assessment forms, dedicated approval workflows with multiple approvers, and a detailed audit trail of activities can help users effectively collaborate in the risk assessment process.
Root Cause Investigation
Identifying the root cause is the next crucial step in preventing the recurrence of such issues. BizPortals CAPA management software, with robust document control and collaborative features, ensures that users have access to all the CAPA records, audit logs, and documentation ready for the root cause analysis.
Trend Analysis
Analyzing previous CAPA trends by studying detailed statistics and metrics helps outline more concrete corrective actions. BizPortals QMS's dedicated CAPA dashboard displays key metrics based on recurring issues, potential risks, and key categories like monthly trends, departments, stages, priority, etc., and helps businesses refine their processes.
Corrective Action Planning
A robust corrective action plan is necessary to address the issues based on the root cause assessment and an in-depth trend analysis. Here, automated review and approval workflow, centralized quality resources, and cross departmental collaboration offered by SharePoint-based CAPA management software can be game changers.
Implementing CAPA
Seamless execution and timely monitoring of the action plan decide the success of the overall corrective and preventive actions. This requires uninterrupted communication across departments, robust task management, progress tracking, and cross-module integration to ensure the plan is executed promptly and efficiently.
Take Quality Further with BizPortals QMS
See how a complete QMS streamlines quality processes across your entire organization.
How Different Industries Apply CAPA Management Software for Smarter Quality Control

Whether in food production or any regulated industry, a SharePoint-based QMS makes CAPA a proactive, collaborative, and measurable process — turning issues into opportunities for lasting quality improvement.
CAPA Software vs. Manual Process: Side-by-Side Comparison
Get a quick snapshot of how BizPortals QMS outperforms traditional CAPA tracking methods—enabling faster resolutions, stronger compliance, and complete process visibility.
| Key Capability | Manual CAPA Process | BizPortals QMS (CAPA Software) |
|---|---|---|
| Issue Tracking |
 Dispersed records in emails/spreadsheets
Dispersed records in emails/spreadsheets
|
 Centralized, real-time issue logging with full traceability
Centralized, real-time issue logging with full traceability
|
| Workflow Automation |
 Manual coordination; prone to delays
Manual coordination; prone to delays
|
 Automated routing, alerts, and approvals
Automated routing, alerts, and approvals
|
| Audit & Compliance |
 Time-consuming data compilation
Time-consuming data compilation
|
 Built-in audit trails, e-signatures, and templates
Built-in audit trails, e-signatures, and templates
|
| Team Collaboration |
 Siloed updates via email/meetings
Siloed updates via email/meetings
|
 Role-based access + integrated tools
Role-based access + integrated tools
|
| Data Insights |
 Manual gathering; limited visibility
Manual gathering; limited visibility
|
 Dashboards, analytics, trend reporting
Dashboards, analytics, trend reporting
|
End the Gaps in Your CAPA Process for Good
Start solving root causes faster with a connected, automated approach to CAPA
FAQs
What is the primary purpose of corrective and preventive actions (CAPA)?
The primary purpose of Corrective and Preventive Actions (CAPA) is to identify, investigate, and resolve the root cause of quality issues to prevent them from recurrence. CAPA is a regulatory requirement for industries operating in controlled environments, and implementing effective CAPA software can help reduce nonconformities, thereby enhancing overall quality control.
Why do regulated industries need BizPortals QMS’s CAPA management software?
How does the CAPA software ensure regulatory compliance?
Does the CAPA system include a dashboard or reporting tool?
How configurable is the CAPA workflow in BizPortals QMS?
Does the system allow electronic signatures?
Yes, BizPortals QMS’s CAPA management software supports electronic signatures for approvals and validations. You can draw and upload your signature, use Active Directory credentials, or simply use OTP-based authentication to ease your process.












