-
Solutions
- Integration
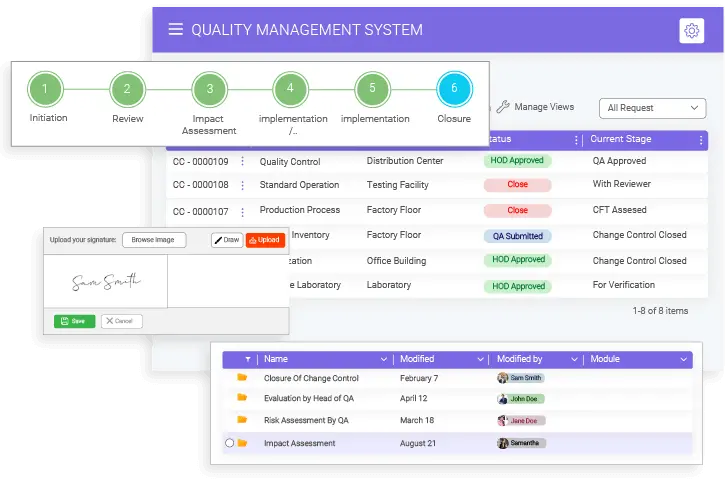
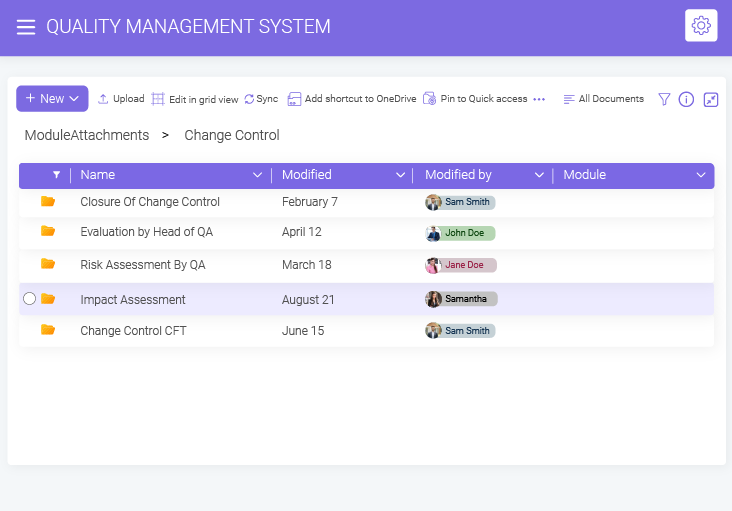
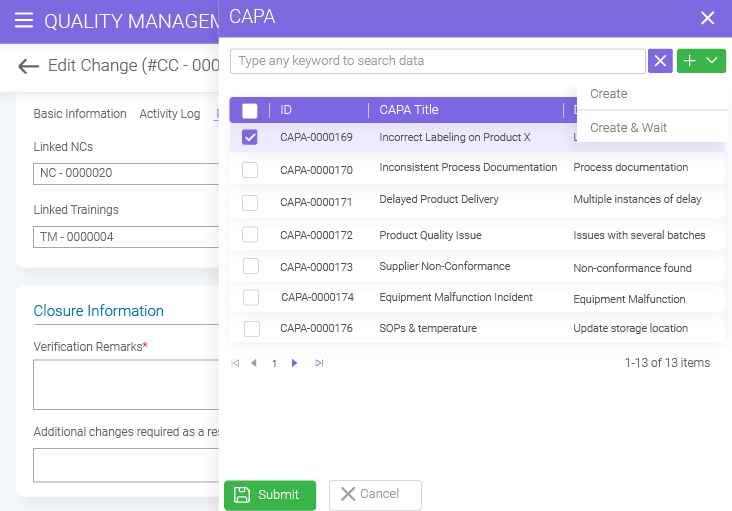
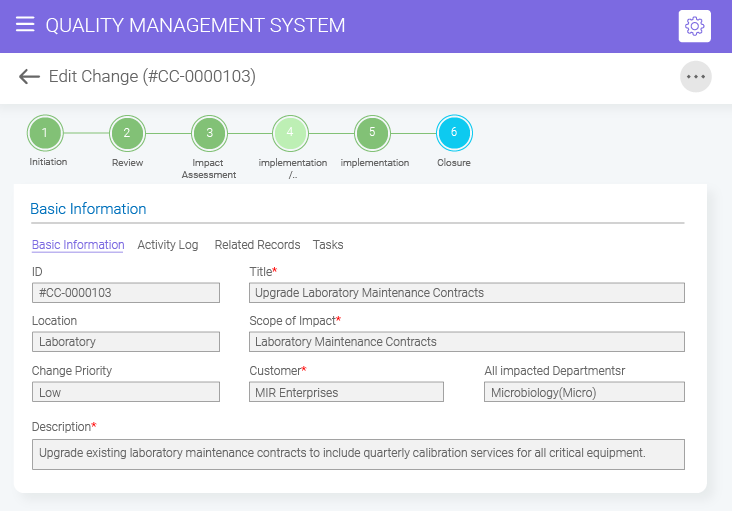
- BizPortals QMS for Acumatica Unifying quality and operations for better results
- Services
- SharePoint Consulting An expert’s roadmap to SharePoint success
- Support and Maintenance Proactive support, enduring performance
- SharePoint Integration A unified intranet for your diverse needs
- Migration Effortless migration, endless SharePoint capabilities
- Custom SharePoint Development Designing a future-ready digital workplace
- Pricing
- Resources
- Blog A treasure trove: Bridging digital expertise
- Brochures Check out our interesting brochure archive
- Guides Resourceful guides: Explore, learn, and thrive
- Success Stories Inspiring success stories of our valued customers
- Videos Unlock valuable insights with our informative videos
- Web Stories Web stories that inform and inspire
- Webinars Discover our upcoming and on-demand webinars
- About
- About Us Empowering businesses with transformative solutions
- Partner Program Partnering for mutual success